What is ITMCTR?
The International Traditional Medicine Clinical Trial Registry (ITMCTR) is a non-profit online register of clinical trials (researches) being conducted in the field of traditional medicine. ITMCTR is a specialized and unique cross-regional registry focusing on the theme of traditional medicine. It is operated by the China Academy of Chinese Medical Sciences and The China Center For Evidence Based Traditional Chinese Medicine. It is recognized as a Primary Registry of WHO in February 2023, and contributes data to the WHO ICTRP.
Registration is voluntary, but certain fields are mandatory when finishing the registration form.
The Sponsor of the clinical trials (researches) should ensure the authenticity of the submitted information, and takes responsibility for the initiation, management and financing of a clinical trial (research). The sponsor may be an individual, or a pharmaceutical company, academic institution, governmental agency, or other organization.


Mission and scope
To cooperate with experts, scholars and academic organizations in the field
To provide dedicated channels for traditional medicine registration
To establish clinical research registration standards
To promote the openness and transparency of traditional medicine clinical research
To help the high-quality output of traditional medicine evidence
To promote traditional medicine to better serve global health
Trials accepted for registration are those conducted in the field of traditional medicine from any country including Chinese medicine, acupuncture, herbal medicine, ayurvida, homeopathy, yonani, complementary and supplementary medicines.
To provide dedicated channels for traditional medicine registration
To establish clinical research registration standards
To promote the openness and transparency of traditional medicine clinical research
To help the high-quality output of traditional medicine evidence
To promote traditional medicine to better serve global health
Trials accepted for registration are those conducted in the field of traditional medicine from any country including Chinese medicine, acupuncture, herbal medicine, ayurvida, homeopathy, yonani, complementary and supplementary medicines.
Standards and criteria
The registration criteria for ITMCTR follow the requirements of the WHO data set.
Unambiguous identification
Quality and Validity
Accessibility
Content
Technical Capacity
Administration and governance

Structure and governance
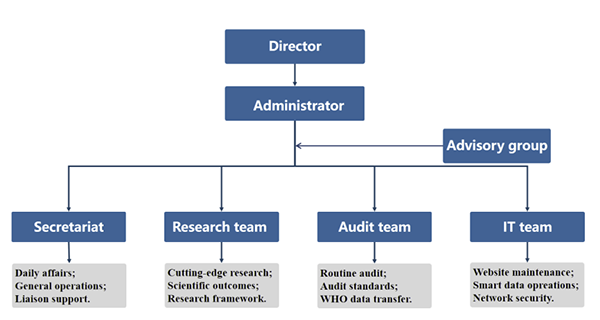
ITMCTR serves as a key initiative to promote the standardization and internationalization of clinical research in traditional medicine. Through a specialized and standardized management system, ITMCTR ensures efficient and effective operations.
Serve as directors, Prof Luqi HUANG, president of the China Academy of Chinese Medical Sciences (CACMS), and Prof Taixiang WU from West China Hospital of Sichuan University, provide strategic guidance and oversee the overall planning and development. Prof Nannan SHI, director of the Division of Research Achievements Management at Institute of Basic Research in Clinical Medicine, CACMS, serves as the operation administrator, responsible for the systematic advancement of daily operations, resource allocation, and coordination.
Four functional departments have also been established, with key responsibilities as follows:
Secretariat
Coordinates daily administrative affairs to ensure the efficient operation of the platform;
Handles meeting arrangements, document management, and issuance of official notices;
Facilitates internal communication and inter-departmental coordination.
Research team
Conducts cutting-edge research related to traditional medicine and provides theoretical and data support;
Compiles research reports, academic papers, and policy recommendations;
Supports the design of research frameworks for clinical trial registration and international cooperation projects.
Audit team
Responsible for routine review of clinical trial registration applications, ensuring data compliance and completeness;
Responsible for developing the internal control audit manual and optimizing audit standards;
Coordinates with WHO for timely transmission and feedback of registration data.
IT team
Maintains the website and platform functionalities to ensure stable registration services;
Integrate artificial intelligence technologies to optimize intelligent data management;
Handles cybersecurity and data backup to enhance platform security.
ITMCTR also establishes an international advisory group responsible for providing strategic recommendations to promote the registration of clinical trials in the field of traditional medicine, as well as to enhance accountability and transparency in clinical trials and the dissemination of their results.
Serve as directors, Prof Luqi HUANG, president of the China Academy of Chinese Medical Sciences (CACMS), and Prof Taixiang WU from West China Hospital of Sichuan University, provide strategic guidance and oversee the overall planning and development. Prof Nannan SHI, director of the Division of Research Achievements Management at Institute of Basic Research in Clinical Medicine, CACMS, serves as the operation administrator, responsible for the systematic advancement of daily operations, resource allocation, and coordination.
Four functional departments have also been established, with key responsibilities as follows:
Secretariat
Coordinates daily administrative affairs to ensure the efficient operation of the platform;
Handles meeting arrangements, document management, and issuance of official notices;
Facilitates internal communication and inter-departmental coordination.
Research team
Conducts cutting-edge research related to traditional medicine and provides theoretical and data support;
Compiles research reports, academic papers, and policy recommendations;
Supports the design of research frameworks for clinical trial registration and international cooperation projects.
Audit team
Responsible for routine review of clinical trial registration applications, ensuring data compliance and completeness;
Responsible for developing the internal control audit manual and optimizing audit standards;
Coordinates with WHO for timely transmission and feedback of registration data.
IT team
Maintains the website and platform functionalities to ensure stable registration services;
Integrate artificial intelligence technologies to optimize intelligent data management;
Handles cybersecurity and data backup to enhance platform security.
ITMCTR also establishes an international advisory group responsible for providing strategic recommendations to promote the registration of clinical trials in the field of traditional medicine, as well as to enhance accountability and transparency in clinical trials and the dissemination of their results.
Advisory group
Advisory Group of ITMCTR is composed of 16 experts, who are from China, Brazil, Malaysia, Korea, Pakistan, Germany, Austria and Japan.
The mission of Advisory Group is to provide suggestions for the significant strategic development of the platform and to promote the registration of clinical trials in the field of traditional medicine.
Members of the Advisory Group